
PPT Evidences of Evolution
Price: 20



PPT Evidences of Evolution
Price: 20

PPT Theories of Evolution
Price: 20

Worksheet: Identifying Human Species by Skull Characteristics
Price: 10

PPT Taxonomy and Classification of Organisms
Price: 20


| Resource type | Worksheet |
| Recommended age | 13 - 18 years |
| File information | docx, 4 pages, 69.8 KB |


| Resource type | Assessment |
| Recommended age | 13 - 18 years |
| File information | docx, 3 pages, 496 KB |
There are no comments yet, write one yourself!

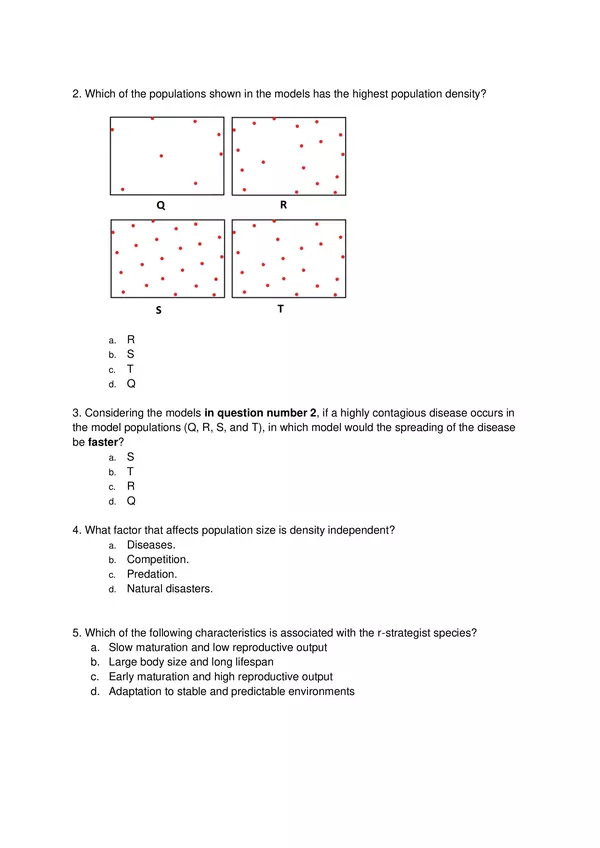
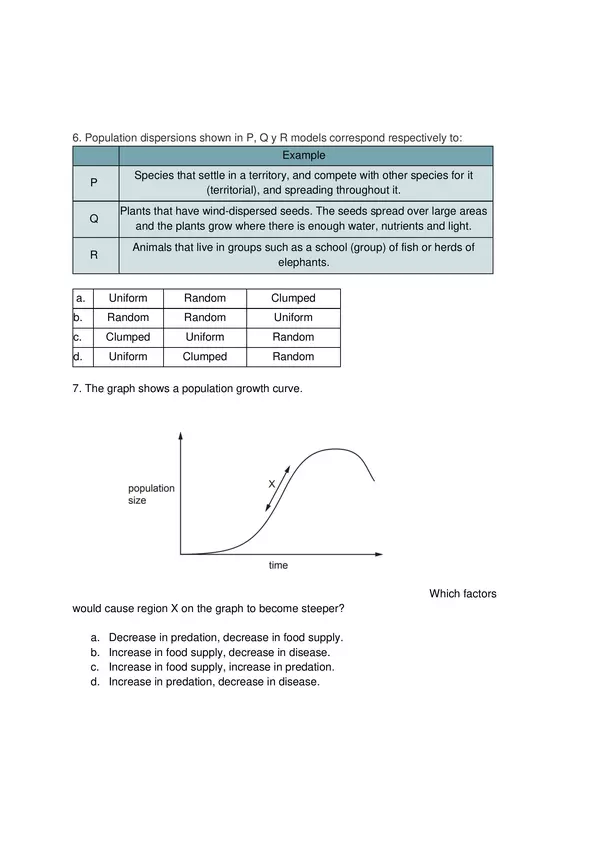
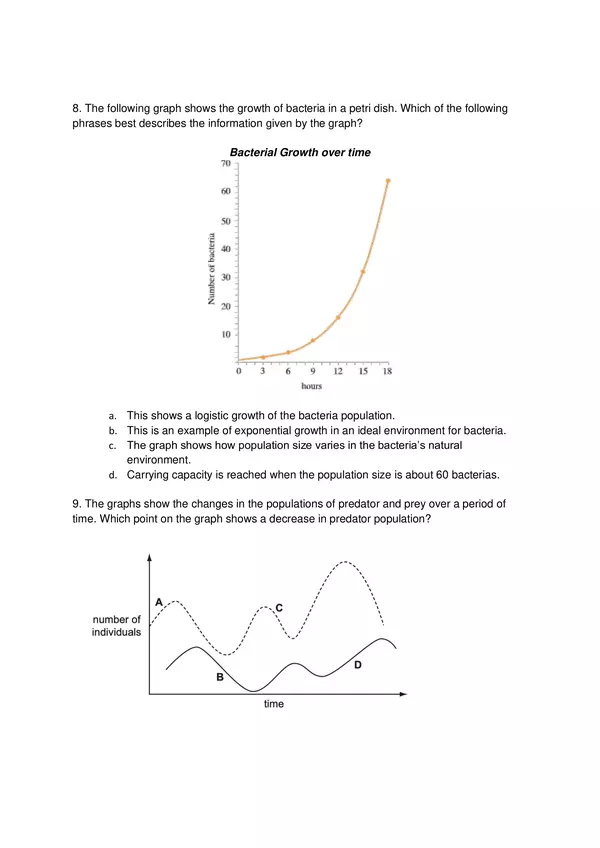
| Resource type | Assessment |
| Recommended age | 13 - 18 years |
| File information | docx, 9 pages, 1.79 MB |
There are no comments yet, write one yourself!
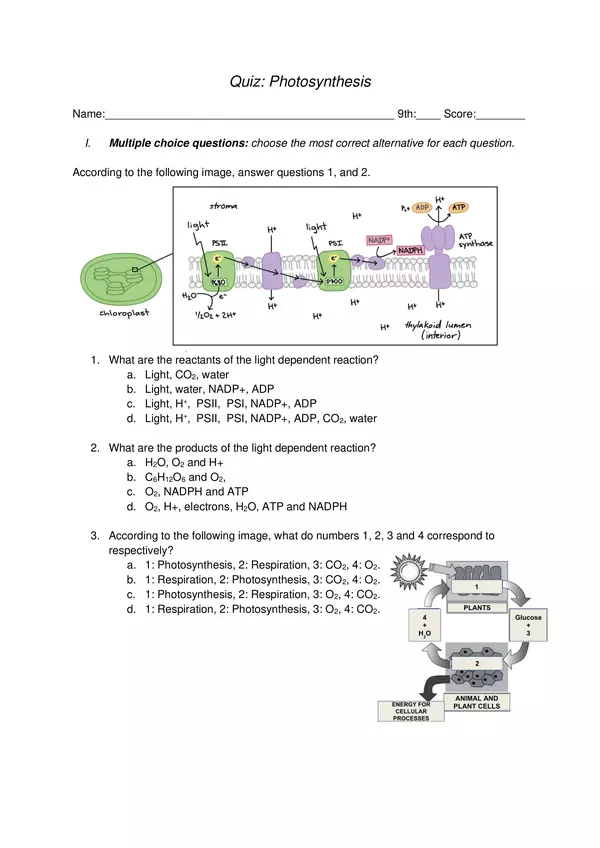
| Resource type | Assessment |
| Recommended age | 14 - 18 years |
| File information | docx, 2 pages, 512 KB |
There are no comments yet, write one yourself!
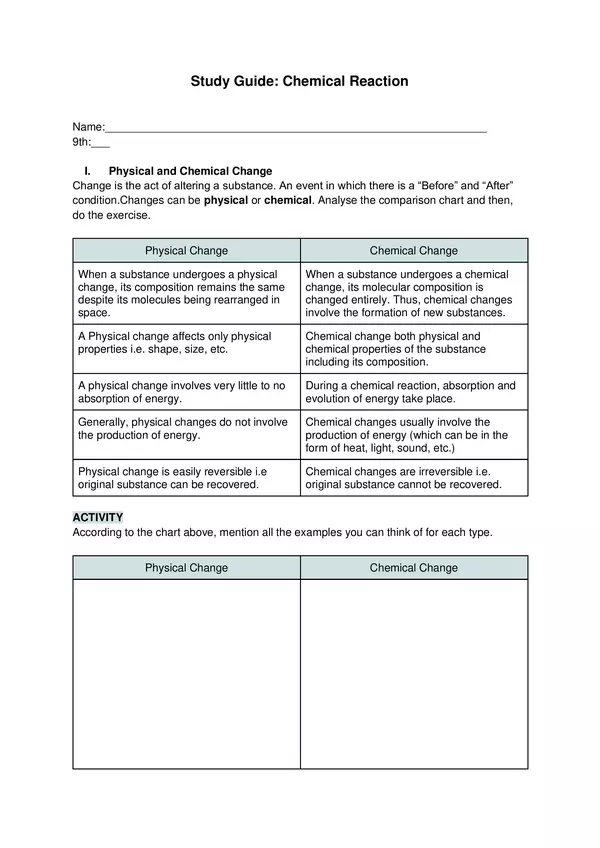

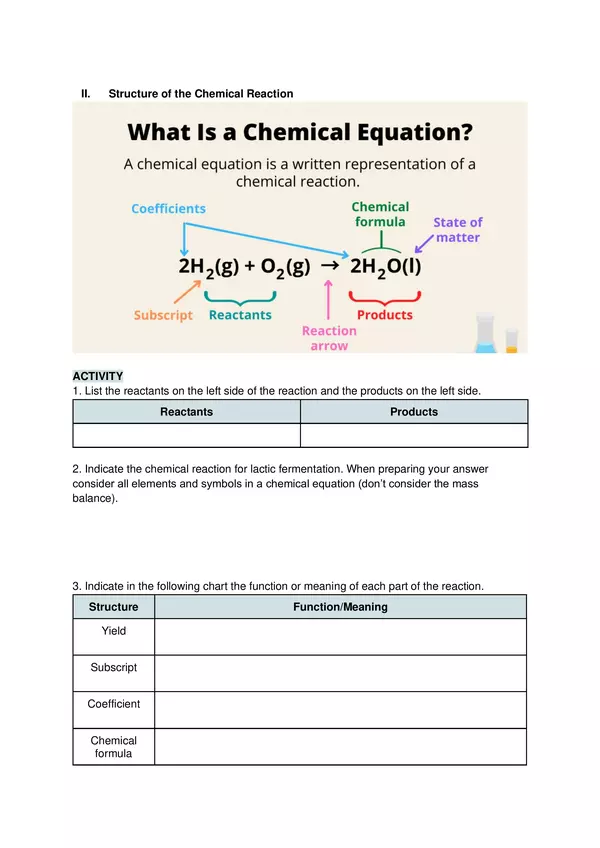

| Resource type | Worksheet |
| Recommended age | 13 - 18 years |
| File information | docx, 10 pages, 2.14 MB |
There are no comments yet, write one yourself!


| Resource type | Worksheet |
| Recommended age | 13 - 18 years |
| File information | docx, 4 pages, 8.63 KB |
There are no comments yet, write one yourself!

| Resource type | Socialemotional development |
| Recommended age | 9 - 18 years |
| File information | pdf, 1 pages, 7.4 MB |
There are no comments yet, write one yourself!

| Resource type | Socialemotional development |
| Recommended age | 9 - 18 years |
| File information | pdf, 1 pages, 201 KB |
There are no comments yet, write one yourself!

| Resource type | Socialemotional development |
| Recommended age | 8 - 18 years |
| File information | pdf, 2 pages, 1.28 MB |
There are no comments yet, write one yourself!




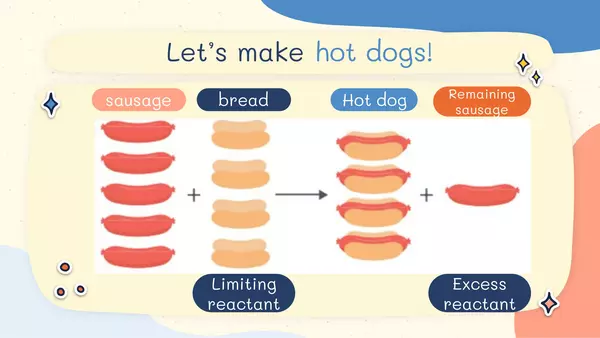
| Resource type | Lesson |
| Recommended age | 13 - 18 years |
| File information | pptx, 14 pages, 1.82 MB |
There are no comments yet, write one yourself!
There are no comments yet, write one yourself!